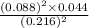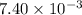Chemistry, 12.10.2019 03:10, shradhwaip2426
At a certain temperature, 0.760 mol so3 is placed in a 2.50 l container. 2so3(g)↽−−⇀2so2(g)+o2(g) at equilibrium, 0.110 mol o2 is present. calculate kc.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, peaceouthjkdrb2398
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 03:30, acaciacoats
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
Do you know the correct answer?
At a certain temperature, 0.760 mol so3 is placed in a 2.50 l container. 2so3(g)↽−−⇀2so2(g)+o2(g) at...
Questions in other subjects:

English, 20.03.2021 21:50

Mathematics, 20.03.2021 21:50


Mathematics, 20.03.2021 21:50




Mathematics, 20.03.2021 21:50


Social Studies, 20.03.2021 21:50


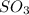 = 0.760 mol
= 0.760 mol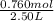
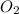 present at equilibrium = 0.110 mol
present at equilibrium = 0.110 mol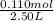
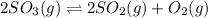
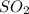 = 2x
= 2x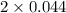
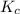 ) as follows.
) as follows.![K_{c} = \frac{[SO_{2}]^{2}[O_{2}]}{[SO_{3}]^{2}}](/tpl/images/0312/0764/e07bc.png)