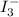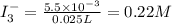The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a known concentration of s₂o₂⁻³(aq) (thiosulfate ion). the determination is based on the net ionic equation 2s₂o₃²⁻(aq)+i₃⁻(aq)⟶s₄o₆²⁻(aq)+3i⁻( aq). given that it requires 38.1 ml of 0.440 m na₂s₂o₃(aq) to titrate a 25.0 ml sample of i₃⁻(aq), calculate the molarity of i₃⁻(aq) in the solution.

Answers: 2
Similar questions

Chemistry, 25.07.2019 22:20, nataluarenhg6924
Answers: 2

Chemistry, 10.09.2019 18:20, AthenAt5607
Answers: 1

Chemistry, 18.10.2019 17:10, Lydiaxqueen
Answers: 1

Chemistry, 19.10.2019 00:30, laequity7325
Answers: 2
Do you know the correct answer?
The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a know...
Questions in other subjects:


Chemistry, 15.06.2021 23:00


English, 15.06.2021 23:00

Health, 15.06.2021 23:00

English, 15.06.2021 23:00




Physics, 15.06.2021 23:00


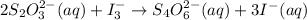
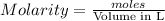
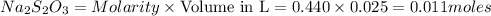
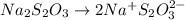
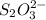 = 0.011
= 0.011 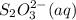 require 1 mole of
require 1 mole of 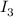
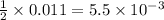 moles of
moles of