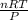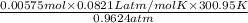Ozzie wanted to do another experiment with a stronger h₂o₂ solution to check the accuracy of the experiment by calculating the theoretical volume of o₂(g) it would produce. then he could compare his experimental volume of o₂(g) to the theoretical volume of o₂(g). he used 4.20 ml of 2.57 m h₂o₂ and the partial pressure of o₂ was 0.9624 atm and the temperature was 300.95 k. what volume of o₂(g) could he theoretically produce (in ml)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, jaejaeJae9534
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1


Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2
Do you know the correct answer?
Ozzie wanted to do another experiment with a stronger h₂o₂ solution to check the accuracy of the exp...
Questions in other subjects:

Computers and Technology, 22.06.2019 21:00

Biology, 22.06.2019 21:00

Biology, 22.06.2019 21:00





Mathematics, 22.06.2019 21:00


English, 22.06.2019 21:00


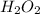 is as follows.
is as follows.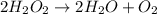
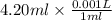
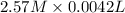
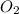 .
.