
Chemistry, 11.10.2019 22:30, tahiratnoel20
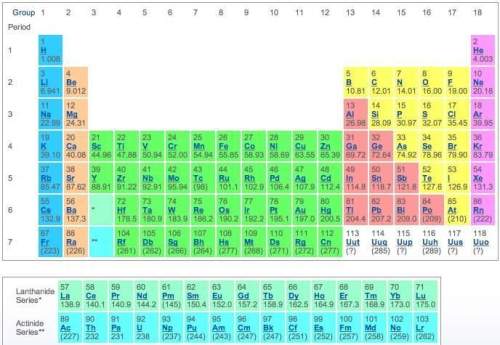
using only the information available in the periodic table, consider the elements potassium and bromine. from their location on the periodic table, identify the oxidation state and number of valence electrons for potassium and bromine. then use this information to describe their reactivity.
which statement most accurately describes the compound formed by potassium and bromine?
a) potassium and bromide form an ionic compound called potassium bromide (kbr).
b) potassium and bromide form an ionic compound called potassium bromide (k2br).
c) potassium and bromide share electrons to form a covalent compound called potassium monobromide (kbr).
d) potassium and bromide share electrons to form a covalent compound called potassium dibromide (kbr2).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3


Chemistry, 23.06.2019 02:40, sherlock19
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
Do you know the correct answer?
using only the information available in the periodic table, consider the elements potassium and brom...
Questions in other subjects:


Mathematics, 18.03.2021 03:20



History, 18.03.2021 03:20





Mathematics, 18.03.2021 03:20






