
Chemistry, 11.10.2019 22:30, adhanom1271
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium chloride and iron. 3mg(s) + 2fecl₃(s) → 3mgcl₂(s) + 2fe(s) a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
Do you know the correct answer?
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium...
Questions in other subjects:

English, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30


Social Studies, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30

English, 26.08.2019 03:30

History, 26.08.2019 03:30

History, 26.08.2019 03:30

History, 26.08.2019 03:30


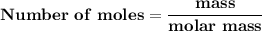
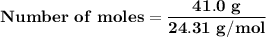
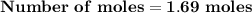
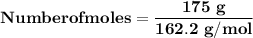
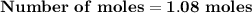
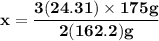 x = 39.34 grams
x = 39.34 grams



