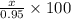Chemistry, 11.10.2019 22:30, kimsouther2
Amixture of kclo3 and kcl with a mass of 0.950 g was heated to produce o2. after heating, the mass of residue was 0.820 g. assuming all the kclo3 decomposed to kcl and o2, calculate the mass percent of kclo3 in the original mixture.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
Do you know the correct answer?
Amixture of kclo3 and kcl with a mass of 0.950 g was heated to produce o2. after heating, the mass o...
Questions in other subjects:

Business, 06.04.2021 19:00

Biology, 06.04.2021 19:00

Mathematics, 06.04.2021 19:00



Mathematics, 06.04.2021 19:00

Mathematics, 06.04.2021 19:00




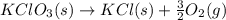
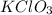 = 122.5 g/mol
= 122.5 g/mol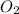 = 32 g/mole
= 32 g/mole
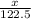
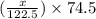
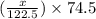 = 0.820 g
= 0.820 g