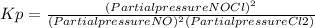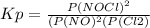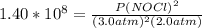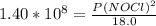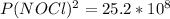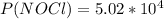Chemistry, 11.10.2019 17:30, BatmanVS1944
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no and 2.0 atm of cl2(g). what is the pressure of no(g) when equilibrium is reached?
3.6 × 10-4 atm
3.8 × 10-8 atm
0.5 atm
2.0 atm
1.0 atm
1.1 × 10-7 atm

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, Britny2386
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3


Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
Do you know the correct answer?
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no...
Questions in other subjects:


Social Studies, 17.11.2019 00:31





Social Studies, 17.11.2019 00:31

Social Studies, 17.11.2019 00:31