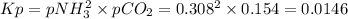nh4co2nh2< > 2nh3(g) + co2(g)


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Do you know the correct answer?
Ammonium carbonate nh4co2nh2 decomposes as follows
nh4co2nh2< > 2nh3(g) + co2(g)
nh4co2nh2< > 2nh3(g) + co2(g)
Questions in other subjects:


Chemistry, 25.04.2020 05:11




Mathematics, 25.04.2020 05:11

Physics, 25.04.2020 05:11



Social Studies, 25.04.2020 05:11