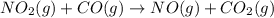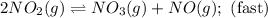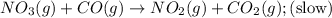Chemistry, 11.10.2019 04:00, aurelio1121
Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon dioxide. no2(g) + co(g) ⟶ no(g) + co2(g) a proposed mechanism for this reaction is 2no2(g) ⟶ no3(g) + no(g) (fast, equilibrium) no3(g) + co(g) ⟶ no2(g) + co2(g) (slow) what is a rate law that is consistent with the proposed mechanism?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
Do you know the correct answer?
Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon dioxide. no2(g)...
Questions in other subjects:



Mathematics, 26.09.2021 15:30

Mathematics, 26.09.2021 15:30

Computers and Technology, 26.09.2021 15:30

Mathematics, 26.09.2021 15:30

Computers and Technology, 26.09.2021 15:30


Computers and Technology, 26.09.2021 15:30


![\text{Rate}=k[NO_3][CO]](/tpl/images/0309/1117/3ba68.png)