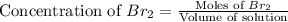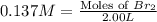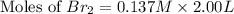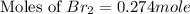Chemistry, 11.10.2019 03:00, simiyi1983
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen bromide: h2 (g) + br2 (g) ↔ 2hbr (g) a mixture of 0.682 mol of h2 and 0.440 mol of br2 is combined in a reaction vessel with a volume of 2.00 l. at equilibrium at 700 k, there are 0.516 mol of h2 present. at equilibrium, there are mol of br2 present in the reaction vessel.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1
Do you know the correct answer?
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen...
Questions in other subjects:







Mathematics, 25.07.2021 20:20

History, 25.07.2021 20:30


Mathematics, 25.07.2021 20:30


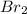 at equilibrium is 0.274 mole.
at equilibrium is 0.274 mole.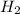 and
and 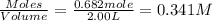
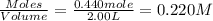
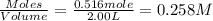
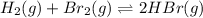
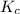 will be,
will be,![K_c=\frac{[HBr]^2}{[H_2][Br_2]}](/tpl/images/0308/9786/3b8cb.png)