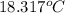Aquantity of 85.0 ml of 0.900 m hcl is mixed with 85.0 ml of 0.900 m koh in a constantpressure calorimeter that has a heat capacity of 325 j/°c. if the initial temperatures of both solutions are the same at 18.24°c, what is the final temperature of the mixed solution? the heat of neutralization is −56.2 kj/mol. assume the density and specific heat of the solutions are the same as those for water.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
Do you know the correct answer?
Aquantity of 85.0 ml of 0.900 m hcl is mixed with 85.0 ml of 0.900 m koh in a constantpressure calor...
Questions in other subjects:



Mathematics, 18.03.2021 02:40

Biology, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Spanish, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40



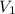 = 85.0 ml,
= 85.0 ml, 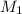 = 0.9 M
= 0.9 M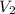 = 85.0 ml,
= 85.0 ml, 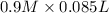 (as 1 L = 1000 ml so, 85 ml = 0.085 L)
(as 1 L = 1000 ml so, 85 ml = 0.085 L)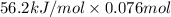
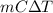
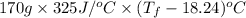
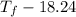
 =
=