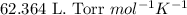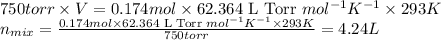Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of a powder. when the mixture is poured down a clogged drain, the following redox reactions occurs: 2naoh(aq) + 2 al(s) + 6h2o(l) → 2naal(oh)4(aq) + 3 h2(g) the heat generated in this reaction melt away grese and the hydrogen gas released stirs up the solids clagging the drain. calculate the volume hydrogen gas formed at 20. ºc and 750. torr if 3.12 g of al is treated with excess naoh.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, brookemcelhaney
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
Do you know the correct answer?
Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of...
Questions in other subjects:

Mathematics, 20.05.2021 23:10



Medicine, 20.05.2021 23:10


Mathematics, 20.05.2021 23:10


Arts, 20.05.2021 23:10



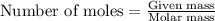
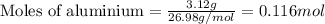
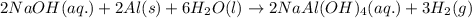
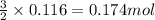 of hydrogen gas
of hydrogen gas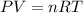
![20^oC=[20+273]K=293K](/tpl/images/0308/3868/3b5d4.png)