
Chemistry, 11.10.2019 00:00, jackfrost5
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.0020 m kscn . the standard solution had an absorbance of 0.480 . fe3+(aq)+scn−(aq)↽−−⇀fescn2+(aq) a trial solution was made in a similar manner, but with a more dilute fe(no3)3 reagent. the initial scn− concentration, immediately after mixing, was 0.00050 m . this trial solution had absorbance of 0.220 . what is the equilibrium concentration of scn− in the trial solution?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, jaejaeJae9534
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
Do you know the correct answer?
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.00...
Questions in other subjects:

Mathematics, 09.09.2020 21:01

Social Studies, 09.09.2020 21:01

English, 09.09.2020 21:01

French, 09.09.2020 21:01

English, 09.09.2020 21:01

Mathematics, 09.09.2020 21:01

English, 09.09.2020 21:01

Mathematics, 09.09.2020 21:01

Biology, 09.09.2020 21:01

Biology, 09.09.2020 21:01


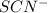 in the trial solution is
in the trial solution is 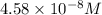
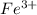 and
and 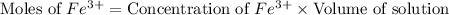
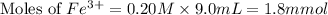
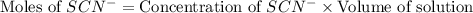
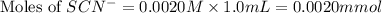
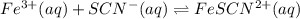
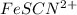
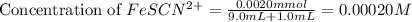

 = molar absorptivity coefficient
= molar absorptivity coefficient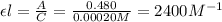
![[SCN^-]_{eqm}=[SCN^-]_{initial}-[FeSCN^{2+}]](/tpl/images/0308/4929/92d10.png)
![[SCN^-]_{initial}](/tpl/images/0308/4929/bc58d.png) = 0.00050 M
= 0.00050 M![[FeSCN^{2+}]](/tpl/images/0308/4929/797d4.png) .
.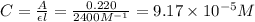
![[SCN^-]_{eqm}=(0.00050M)-(9.17\times 10^{-5}M)](/tpl/images/0308/4929/c2881.png)
![[SCN^-]_{eqm}=4.58\times 10^{-8}M](/tpl/images/0308/4929/1e6f2.png)




