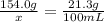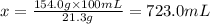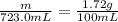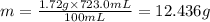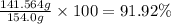The solubility of solid w in water is: 1.72 g/100 ml at 0°c, 21.3/100 ml at 100°c. a) how many ml of boiling water are required to dissolve 154.0 g of w? (report to the nearest ml) if solution were cooled to 0°c, how many grams of w would crystallize out? (report to one decimal place) b) what is the percent recovery? (report to one decimal place) (show calculations)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Do you know the correct answer?
The solubility of solid w in water is: 1.72 g/100 ml at 0°c, 21.3/100 ml at 100°c. a) how many ml o...
Questions in other subjects:

History, 26.12.2019 02:31

Mathematics, 26.12.2019 02:31


History, 26.12.2019 02:31

Physics, 26.12.2019 02:31

Mathematics, 26.12.2019 02:31