
Chemistry, 10.10.2019 05:30, Queenbee2304
Calculate the radius of tantalum (ta) atom, given that ta has a bcc crystal structure, a density of 16.6 g/cm, and an atomic weight of 180.9 g/mol. (avogadro number, 6.023 x 103 atoms/mol).

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Do you know the correct answer?
Calculate the radius of tantalum (ta) atom, given that ta has a bcc crystal structure, a density of...
Questions in other subjects:

Mathematics, 08.08.2021 21:30


Mathematics, 08.08.2021 21:30

Mathematics, 08.08.2021 21:30

Mathematics, 08.08.2021 21:30



Mathematics, 08.08.2021 21:30

Mathematics, 08.08.2021 21:30

Biology, 08.08.2021 21:30


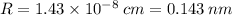
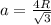
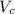 is
is 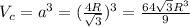
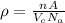
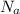 = Avogadro’s number (
= Avogadro’s number (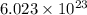 atoms/mol)
atoms/mol)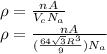
![\rho=\frac{nA}{(\frac{64\sqrt{3}R^3}{9})N_{a}}\\\frac{64\sqrt{3}R^3}{9}=\frac{nA}{\rho N_{a}}\\R^3=\frac{nA}{\rho N_{a}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}} \\R=\sqrt[3]{\frac{nA}{\rho N_{a}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}}}](/tpl/images/0306/0375/e96f7.png)
![R=\sqrt[3]{\frac{2\cdot 180.9}{16.6\cdot 6.023 \times 10^{23}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}}}\\R = 1.43 \times 10^{-8} \:cm = 0.143 \:nm](/tpl/images/0306/0375/377b1.png)




