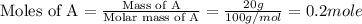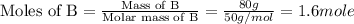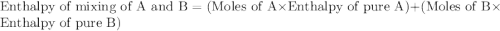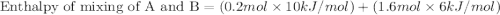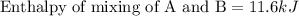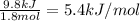Chemistry, 10.10.2019 06:00, cheerleaderautumnche
A20 wt% a solution is obtained by mixing a component with b in an insulated mixer at steady state. for every mole of solution 1 kj is removed to keep the system temperature constant. determine the enthalpy of mixture for this solution. molecular weight of a: 100 g/mol; molar enthalpy of pure a: 10 kj/mol molecular weight of b: 50 g/mol; molar enthalpy of pure b: 6 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, YatesDevon3371
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
Do you know the correct answer?
A20 wt% a solution is obtained by mixing a component with b in an insulated mixer at steady state. f...
Questions in other subjects:

History, 05.10.2019 00:30

Mathematics, 05.10.2019 00:30




History, 05.10.2019 00:30


Chemistry, 05.10.2019 00:30