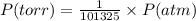Chemistry, 10.10.2019 05:20, hillarytrinh
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, are injected into an evacuated 536 cm3 flask.
a. find the number of moles of oxygen present prior to mixing the gases, assuming the temperature remains constant, and that oxygen is an ideal gas. (5pts)
b. find the number of moles of nitrogen present prior to mixing the gases, assuming the temperature remains constant and that nitrogen is an ideal gas. (5pts)
c. find the total pressure of oxygen and nitrogen in the flask (after the two gases are mixed together.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 23.06.2019 13:30, jcastronakaya
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3
Do you know the correct answer?
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, a...
Questions in other subjects:

Mathematics, 29.11.2019 10:31

English, 29.11.2019 10:31

Mathematics, 29.11.2019 10:31



Mathematics, 29.11.2019 10:31

Mathematics, 29.11.2019 10:31



History, 29.11.2019 10:31