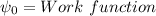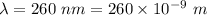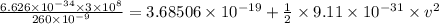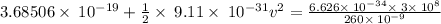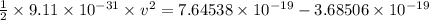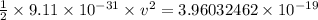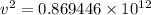Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 11:50, natorihill629
Charles's law describes the relationship of the volume and temperature of gas at a constant mass and pressure. according to this law, what would happen to the temperature of the gas if its volume decreased from 1.0 l to 0.50 l?
Answers: 3

Chemistry, 23.06.2019 15:00, ericperkins10ox0b27
In two or more complete sentences describe all of the van der waals forces that exist between molecules of sulfur dioxide, so2.
Answers: 1
Do you know the correct answer?
The work function for metallic potassium is 2.3 ev. calculate the velocity in km/s for electrons eje...
Questions in other subjects:





Mathematics, 07.09.2019 01:20

History, 07.09.2019 01:20


Mathematics, 07.09.2019 01:20

History, 07.09.2019 01:20


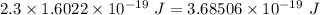
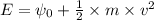
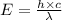
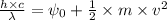
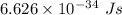
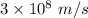
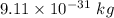
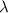 is the wavelength of the light being bombarded
is the wavelength of the light being bombarded