
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
e5 = -7 x 10-19 j
e4 = -11 x 10-19 j
e3 = -15 x 10-19 j
e2 = -17 x 10-19 j
e1 = -20 x 10-19 j
(this is not an actual atom that exists. according to the bohr model, energies should follow the expression discussed in section 6.2 of silberberg. this exercise prepares you for calculations like questions 6 and 7 of problem set 1.)
(a) if the electron is initially in the n = 4 level, what is the shortest wavelength of radiation that could be emitted? provide your answer to one significant figure: -7 m.
(b) what is the ionization energy (in kj/mol) of the atom in its first excited state? provide your answer to two significant figures: 103 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2


Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2

Chemistry, 23.06.2019 13:00, kayleegeise
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
Do you know the correct answer?
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
...
e6 = -2 x 10-19 j
...
Questions in other subjects:




Mathematics, 03.12.2019 03:31


Mathematics, 03.12.2019 03:31



Mathematics, 03.12.2019 03:31


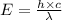
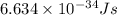
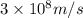
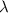 = Wavelength of the radiation
= Wavelength of the radiation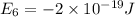
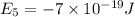
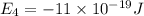
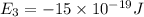
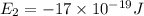
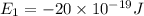
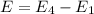
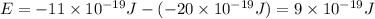
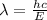
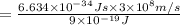
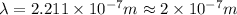
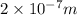 is the shortest wavelength of radiation that could be emitted.
is the shortest wavelength of radiation that could be emitted.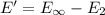
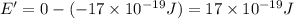
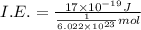
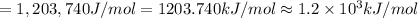
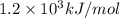 is the ionization energy of the atom in its first excited state.
is the ionization energy of the atom in its first excited state.




