
Chemistry, 10.10.2019 05:00, akaeiraspruell
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to carbonate (co32-) to determine: hco3 = co2 + h+ k = 10-10.33 (a) whether hco3 or co32- would dominate at ph 9.1 and (b) what the concentration of [co32-] would be at this ph if [hco3 ] = 10-6 m

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
Do you know the correct answer?
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to...
Questions in other subjects:


History, 15.12.2019 01:31

Mathematics, 15.12.2019 01:31


Mathematics, 15.12.2019 01:31

Mathematics, 15.12.2019 01:31


Mathematics, 15.12.2019 01:31

Mathematics, 15.12.2019 01:31

History, 15.12.2019 01:31


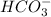 will dominate at pH = 9.1
will dominate at pH = 9.1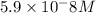
![pH=-\log[H^+]](/tpl/images/0305/9437/cf945.png) ......(1)
......(1)![9.1=-\log[H^+]](/tpl/images/0305/9437/e5ddb.png)
![[H^+]=10^{-9.1}](/tpl/images/0305/9437/b7f9d.png)
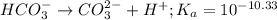
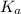 for above reaction follows:
for above reaction follows:![K_a=\frac{[CO_3^{2-}]\times [H^+]}{[HCO_3^-]}](/tpl/images/0305/9437/fea1b.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{[HCO_3^-]}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=\frac{10^{-9.1}}{10^{-10.33}}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=16.98](/tpl/images/0305/9437/786f2.png)
![[HCO_3^-]=16.98\times [CO_3^{2-}]](/tpl/images/0305/9437/e6ce5.png)
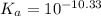
![[HCO_3^-]=10^{-6}M](/tpl/images/0305/9437/1335d.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{10^{-6}}](/tpl/images/0305/9437/27401.png)
![[CO_3^{2-}]=\frac{10^{-6}\times 10^{-10.33}}{10^{-9.1}}=5.9\times 10^-8}M](/tpl/images/0305/9437/65347.png)




