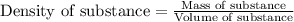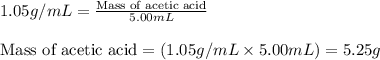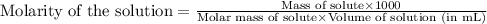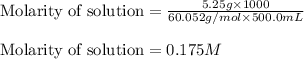"pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity of a solution prepared by dissolving 5.00 ml of glacial acetic acid at 25 °c in sufficient water to give 500.0 ml of solution. the density of glacial acetic acid at 25 °c is 1.05 g/ml. pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity of a solution prepared by dissolving 5.00 ml of glacial acetic acid at 25 °c in sufficient water to give 500.0 ml of solution. the density of glacial acetic acid at 25 °c is 1.05 g/ml.
a. 3.50 × 10-5 m
b. 126 m
c. 0.0350 m
d. 2.10 m
e. 2.10 × 10-3 m

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 05:30, choatefarmsus
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 13:30, jcastronakaya
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3
Do you know the correct answer?
"pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity...
Questions in other subjects:

History, 30.03.2020 21:45

Mathematics, 30.03.2020 21:45








Mathematics, 30.03.2020 21:45