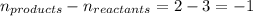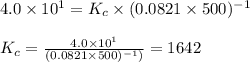The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three gases has partial pressures of 9.10×10−2 atm , 0.174 atm , and 0.24 atm for no, cl2, and nocl, respectively. part apart complete calculate kp for this reaction at 500.0 k. express your answer using two significant figures. kp = 40 previous answers correct part b if the vessel has a volume of 6.00 l , calculate kc at this temperature. express your answer using two significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1


Do you know the correct answer?
The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three...
Questions in other subjects:

History, 19.10.2021 09:30


Mathematics, 19.10.2021 09:30

Mathematics, 19.10.2021 09:30



History, 19.10.2021 09:30

Mathematics, 19.10.2021 09:30

Mathematics, 19.10.2021 09:30

Mathematics, 19.10.2021 09:30


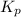 for the given reaction is
for the given reaction is 
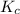 for the given reaction is 1642.
for the given reaction is 1642.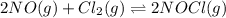
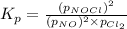
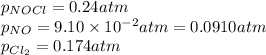
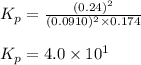
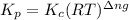
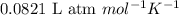
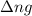 = change in number of moles of gas particles =
= change in number of moles of gas particles =