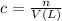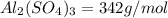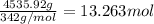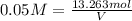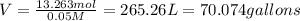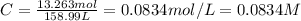Chemistry, 10.10.2019 03:20, Mrdwarf7163
You have an-empty 42-gatbarrel-and-a 10-lb bag of dry alum, al2(so4). (a) calculate the number of gallons of 0.05 m solution you can make with this amount of alumthe-materials you hve on hand. (b) suppose you put all the alum into a 42-gal-the barrel and filled it full calculate the molar concentration of the resulting solution

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Kaylinne1181
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2


Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
Do you know the correct answer?
You have an-empty 42-gatbarrel-and-a 10-lb bag of dry alum, al2(so4). (a) calculate the number of ga...
Questions in other subjects:




Biology, 12.03.2020 18:54

Biology, 12.03.2020 18:54


Geography, 12.03.2020 18:54