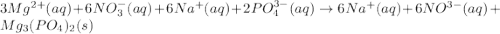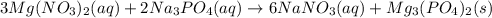When a solution of magnesium nitrate is mixed with a solution of sodium phosphate, the solid that forms is magnesium phosphate. select the equation that represents the complete ionic equation. a. mg(no3)2(aq) + na2po4(aq) mgpo4(s) + nano3(aq) b. mg2+(aq) + po43-(aq) mgpo4(s) c. 3mg(no3)2(aq) + 2na3po4(aq) mg3(po4)2(s) + 6nano3(aq) d. 3mg2+(aq) + 2po43-(aq) mg3(po4)2(s) e. 3mg2+(aq)+6no3-(aq) + 6na+(aq) + 2po43-(aq) mg3(po4)2(s)+6no3-(aq)+ 6na+(aq)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
Do you know the correct answer?
When a solution of magnesium nitrate is mixed with a solution of sodium phosphate, the solid that fo...
Questions in other subjects:

History, 05.02.2020 04:50







History, 05.02.2020 04:50

Mathematics, 05.02.2020 04:50