
Procedure for titrating an acid against a standard solution of naoh. the acid-base indicator, phenolphthalein, is colorless in acidic solution but takes on a pink color in basic solution. how would the volume of standard solution added change if that solution were ba(oh)2(aq) instead of naoh(aq)?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1



Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
Do you know the correct answer?
Procedure for titrating an acid against a standard solution of naoh. the acid-base indicator, phenol...
Questions in other subjects:


Mathematics, 28.05.2020 21:07





English, 28.05.2020 21:07




 solution required will be half the volume required for NaOH solution.
solution required will be half the volume required for NaOH solution. ion upon complete dissociation
ion upon complete dissociation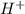 ion. But one molecule of
ion. But one molecule of 



