
For the following reaction, kc = 15 at 700 k. 2 no(g) + cl2(g) ⇄ 2 nocl(g) if we have [no] = 0.15 m, [cl2] = 0.15 m, [nocl] = 0.40 m at 700 k, what will happen? group of answer choices the equilibrium will not shift. the equilibrium will shift to the left, but will use up only part of the nocl. the equilibrium will shift to the right, but will use up only part of the no and cl2. the equilibrium will shift to the right until all the reactants are used up. the equilibrium will shift to the left until all the nocl is used up.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3


Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
Do you know the correct answer?
For the following reaction, kc = 15 at 700 k. 2 no(g) + cl2(g) ⇄ 2 nocl(g) if we have [no] = 0.15 m,...
Questions in other subjects:

Social Studies, 18.10.2019 23:30

Biology, 18.10.2019 23:30

Mathematics, 18.10.2019 23:30

Physics, 18.10.2019 23:30





Mathematics, 18.10.2019 23:30

Mathematics, 18.10.2019 23:30


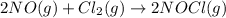
![Q=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0305/1218/afbe9.png)
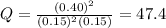
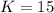
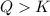 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.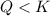 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.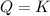 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.



