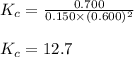At equilibrium, the concentrations of reactants and products can be predicted using the equilibrium constant, kc, which is a mathematical expression based on the chemical equation. for example, in the reaction aa+bb⇌cc+dd where a, b, c, and d are the stoichiometric coefficients, the equilibrium constant is kc=[c]c[d]d[a]a[b]b where [a], [b], [c], and [d] are the equilibrium concentrations. if the reaction is not at equilibrium, the quantity can still be calculated, but it is called the reaction quotient, qc, instead of the equilibrium constant, kc. qc=[c]tc[d]td[a]ta[b]tb where each concentration is measured at some arbitrary time t. part a a mixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.800 m , and [c] = 0.500 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.150 m and [c] = 0.700 m . calculate the value of the equilibrium constant, kc. express your answer numerically.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Do you know the correct answer?
At equilibrium, the concentrations of reactants and products can be predicted using the equilibrium...
Questions in other subjects:


English, 19.04.2021 17:40

Mathematics, 19.04.2021 17:40


Biology, 19.04.2021 17:40

Mathematics, 19.04.2021 17:40

Mathematics, 19.04.2021 17:40

Mathematics, 19.04.2021 17:40

English, 19.04.2021 17:40

Chemistry, 19.04.2021 17:40


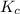
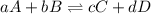
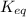 is written as:
is written as:![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0304/8312/9c8b0.png)
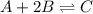
![[A]_{eq}=0.150M](/tpl/images/0304/8312/2394d.png)
![[C]_{eq}=0.700M](/tpl/images/0304/8312/9e4dd.png)
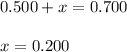
![[B]_{eq}=(0.800-x)=0.800-0.200=0.600M](/tpl/images/0304/8312/eb193.png)
![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0304/8312/240ef.png)