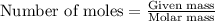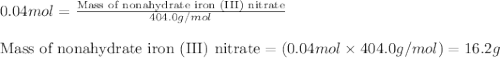In reality, a hydrate of iron(iii) nitrate had to be used, not the anhydrous salt. as you may guess, some of the hydrate’s mass is water, and some is iron(iii) nitrate. how many grams of fe(no3)3•9h2o needed to be dissolved in water to make 2 l of 0.0020 m fe(no3)3? molecular weight of the nonahydrate is 404.0 g/mol. hint: try to set up an equation using x, and solving it. assume that the density of your solution is 1.000 g/ml.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Do you know the correct answer?
In reality, a hydrate of iron(iii) nitrate had to be used, not the anhydrous salt. as you may guess,...
Questions in other subjects:

Mathematics, 10.02.2022 03:30

Business, 10.02.2022 03:30

Mathematics, 10.02.2022 03:30



Spanish, 10.02.2022 03:30





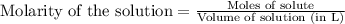
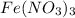 = 0.0020 M
= 0.0020 M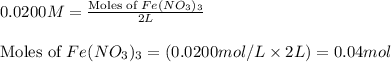
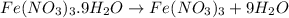
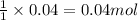 of hydrated iron (III) nitrate
of hydrated iron (III) nitrate