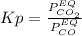Chemistry, 09.10.2019 05:30, minnahelhoor
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s) kp = 20 at 873 k if a reaction vessel at equilibrium contains solid ni, solid nio, 400 mm hg of co2, and 20 mm hg of co, doubling the amount of co(g) present will lead to the production of more solid nickel at 873 k. group of answer choices

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
Do you know the correct answer?
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s)...
Questions in other subjects:


Engineering, 06.07.2019 04:30




Engineering, 06.07.2019 04:30




Mathematics, 06.07.2019 04:30