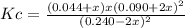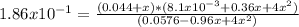Chemistry, 09.10.2019 03:00, hi510hello
Consider 2 nocl(g) \longleftrightarrow⟺ 2 no(g) + cl2 (g) at 25oc under conditions other than equilibrium, there are 1.20 moles of nocl , 0.450 moles of no, and 0.220 moles of cl2 in a 5.00 l flask. kc = 1.86 x 10-1. show work for credit. a. calculate q. b. predict the direction of the reaction. c. calculate equilibrium concentrations of all species present. the equilibrium concentration of cl2 is 0.0490 m.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
Do you know the correct answer?
Consider 2 nocl(g) \longleftrightarrow⟺ 2 no(g) + cl2 (g) at 25oc under conditions other than equili...
Questions in other subjects:

History, 18.04.2020 02:40

Chemistry, 18.04.2020 02:40


Social Studies, 18.04.2020 02:40


Chemistry, 18.04.2020 02:40

Mathematics, 18.04.2020 02:40


Mathematics, 18.04.2020 02:40

Mathematics, 18.04.2020 02:40


![Q = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0302/3128/03e8d.png)
![Q = \frac{[Cl2]x[[NO]^2}{[NOCl]^2}](/tpl/images/0302/3128/de159.png)