
Chemistry, 08.10.2019 21:30, NaVaThEBeAsT
Iron ore can be reduced to iron by the following reaction: fe2o3(s) + 3h2(g) → 2fe + 3h2o(l) (a) how many moles of fe can be made from 1.25 moles of fe2o3? (b) how many moles of h2 are needed to make 3.75 moles of fe? (c) if the reaction yields 12.50 moles of h2o, what mass of fe2o3 was used

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1
Do you know the correct answer?
Iron ore can be reduced to iron by the following reaction: fe2o3(s) + 3h2(g) → 2fe + 3h2o(l) (a) ho...
Questions in other subjects:




English, 07.07.2021 19:30

Mathematics, 07.07.2021 19:30

Mathematics, 07.07.2021 19:30

Mathematics, 07.07.2021 19:30

Business, 07.07.2021 19:30



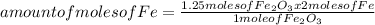
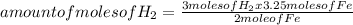
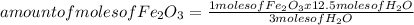
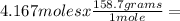 665.4699 grams
665.4699 grams



