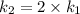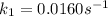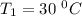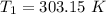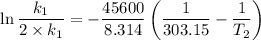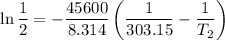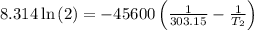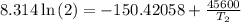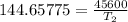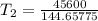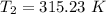Chemistry, 08.10.2019 03:20, jonlandis6
The arrhenius equation shows the relationship between the rate constant k and the temperature t in kelvins and is typically written as k=ae−ea/rt where r is the gas constant (8.314 j/mol⋅k), a is a constant called the frequency factor, and ea is the activation energy for the reaction. however, a more practical form of this equation is lnk2k1=ear(1t1−1t2) which is mathematically equivalent to lnk1k2=ear(1t2−1t1) where k1 and k2 are the rate constants for a single reaction at two different absolute temperatures (t1 and t2). part a the activation energy of a certain reaction is 45.6 kj/mol . at 30 ∘c , the rate constant is 0.0160s−1 . at what temperature in degrees celsius would this reaction go twice as fast

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
Do you know the correct answer?
The arrhenius equation shows the relationship between the rate constant k and the temperature t in k...
Questions in other subjects:

English, 12.01.2021 21:40


Mathematics, 12.01.2021 21:40



Mathematics, 12.01.2021 21:40


Mathematics, 12.01.2021 21:40



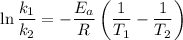
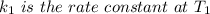
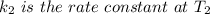
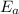 is the activation energy
is the activation energy