
Chemistry, 08.10.2019 02:30, iwantcandy2002
Be sure to answer all parts. sulfur dioxide is released in the combustion of coal. scrubbers use lime slurries of calcium hydroxide to remove the so2 from the flue gases. write the balanced equation for the reaction between solid calcium hydroxide and so2. include the states of all reactants and products in your equation. now, calculate the δs o at 298 k [s o of caso3(s) = 101.4 j/mol k].

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1



Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1
Do you know the correct answer?
Be sure to answer all parts. sulfur dioxide is released in the combustion of coal. scrubbers use lim...
Questions in other subjects:


Physics, 19.07.2020 14:01

Mathematics, 19.07.2020 14:01


Mathematics, 19.07.2020 14:01

Mathematics, 19.07.2020 14:01





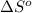 for the reaction is -160.6 J/K
for the reaction is -160.6 J/K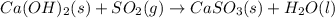
![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{products}]-\sum [n\times \Delta S^o_{reactants}]](/tpl/images/0298/9458/e71e2.png)
![\Delta S^o_{rxn}=[(1\times \Delta S^o_{CaSO_3(s)})+(1\times \Delta S^o_{H_2O(l)})]-[(1\times \Delta S^o_{Ca(OH)_2(s)})+(1\times \Delta S^o_{SO_2(g)})]](/tpl/images/0298/9458/6c149.png)
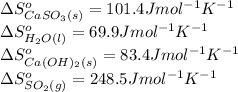
![\Delta S^o_{rxn}=[(1\times (101.4))+(1\times (69.9))]-[(1\times (83.4))+(1\times (248.5))]\\\\\Delta S^o_{rxn}=-160.6J/K](/tpl/images/0298/9458/59a05.png)




