
Chemistry, 08.10.2019 03:00, machucajazz34182
The equilibrium constant, kc, for the reaction h2 (g) + i2 (g) ⇄ 2hi (g) at 425°c is 54.8. a reaction vessel contains 0.0890 m hi, 0.215 m h2, and 0.498 m i2. which statement is correct about this reaction mixture? view available hint(s) the equilibrium constant, kc, for the reaction h2 (g) + i2 (g) ⇄ 2hi (g) at 425°c is 54.8. a reaction vessel contains 0.0890 m hi, 0.215 m h2, and 0.498 m i2. which statement is correct about this reaction mixture? the reaction is not at equilibrium and will proceed to make more reactants to reach equilibrium. the reaction quotient is greater than 1. the reaction is not at equilibrium and will proceed to make more products to reach equilibrium. the reaction mixture is at equilibrium.

Answers: 1
Other questions on the subject: Chemistry



Do you know the correct answer?
The equilibrium constant, kc, for the reaction h2 (g) + i2 (g) ⇄ 2hi (g) at 425°c is 54.8. a reactio...
Questions in other subjects:


Mathematics, 23.04.2021 02:50

Mathematics, 23.04.2021 02:50



Mathematics, 23.04.2021 02:50


Mathematics, 23.04.2021 02:50



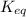
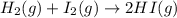
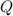 is written as:
is written as:
![Q=\frac{[HI]^2}{[H_2]^1[I_2]^1}](/tpl/images/0298/9983/d3785.png)
![Q=\frac{[0.0890]^2}{[0.215]^1[0.498]^1}](/tpl/images/0298/9983/e2691.png)
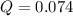
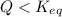 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.



