
Chemistry, 08.10.2019 02:20, SpookyAlex2132
Ook at sample problem 18.12 in the 8th ed silberberg book. write a balanced chemical equation (salt hydrolysis). so acetate ion + water goes to acetic acid + oh-. write the kb expression. notice it is a kb since hydroxide ion is a product. ka is given for acetic acid so calculate the kb for the acetate ion. set up an ice chart. find x which represents hydroxide ion concentration, find poh, convert to ph and enter that to 2 decimal places. what is the ph of 0.35 m sodium acetate solution at 25°c? the ka of acetic acid is 2x10-5.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
Do you know the correct answer?
Ook at sample problem 18.12 in the 8th ed silberberg book. write a balanced chemical equation (salt...
Questions in other subjects:



Mathematics, 25.02.2020 19:56








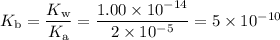
![\dfrac{\text{[HA ][OH$^{-}$]}}{\text{[A$^{-}$]}} = \dfrac{x^{2}}{0.35-x} = 5 \times 10^{-10}](/tpl/images/0298/9039/d19cc.png)
![\dfrac{\text{[HA]}}{K_{\text{b}}} = \dfrac{0.35}{5 \times 10^{-10}} = 7 \times 10^{8} 400\\\\\therefore x \ll 0.35\\\\\dfrac{x^{2}}{0.35} = 5 \times 10^{-10}\\\\x^{2} = 0.35 \times 5 \times 10^{-10} = 1.8\times 10^{-10}\\\\x = \sqrt{1.8\times 10^{-10}} = \mathbf{1 \times 10^{-5}}](/tpl/images/0298/9039/dbd7b.png)




