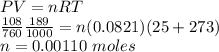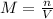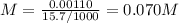The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved in about 60 ml of water and titrated with an naoh solution; 15.7 ml of the naoh solution were required to neutralize the hcl. calculate the molarity of the naoh solution.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
Do you know the correct answer?
The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved...
Questions in other subjects:




Chemistry, 07.09.2019 02:30


Medicine, 07.09.2019 02:30