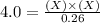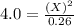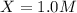Chemistry, 08.10.2019 01:00, hamadehassan
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l container and allowed to come to equilibrium, and the equilibrium concentration of pcl5(g) is 0.26 m, what is the equilibrium concentration of pcl3?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:50, stodd9503
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
Do you know the correct answer?
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l...
Questions in other subjects:





Social Studies, 05.07.2019 20:50

Social Studies, 05.07.2019 20:50



Mathematics, 05.07.2019 20:50


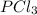 is, 1.0 M
is, 1.0 M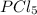 = 0.26 M
= 0.26 M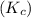 = 4.0
= 4.0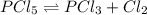
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0298/6790/73fe0.png)
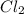 are equal.
are equal.