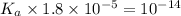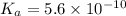Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Do you know the correct answer?
Given that at 25.0 ∘c ka for hcn is 4.9×10−10 and kb for nh3 is 1.8×10−5, calculate kb for cn− and k...
Questions in other subjects:





Mathematics, 06.05.2020 03:38


History, 06.05.2020 03:38


Computers and Technology, 06.05.2020 03:38


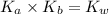
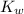 is the dissociation constant of water.
is the dissociation constant of water.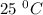 ,
, 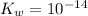
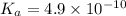
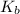 for CN⁻ can be calculated as:
for CN⁻ can be calculated as:
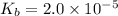
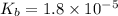
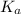 for
for 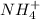 can be calculated as:
can be calculated as: