
If a 0.1 m solution of glucose 1-phosphate is incubated with a catalytic amount of phospho-glucomutase, the glucose 1-phosphate is transformed to glucose 6-phosphate until equilibrium is reached. at equilibrium, the concentration of glucose 1-phosphate is 4.5 x 10–3 m and that of glucose 6-phosphate is 8.6 × 10–2 m. set up the expressions for the calculation of keq' and g'° for this reaction (in the direction of glucose 6-phosphate formation). (r = 8.315 j/mol·k; t = 298 k)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
Do you know the correct answer?
If a 0.1 m solution of glucose 1-phosphate is incubated with a catalytic amount of phospho-glucomuta...
Questions in other subjects:

Mathematics, 08.03.2021 01:00



Chemistry, 08.03.2021 01:00


Social Studies, 08.03.2021 01:00


Spanish, 08.03.2021 01:00


History, 08.03.2021 01:00


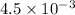 M
M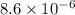 M
M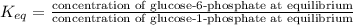
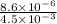
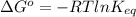
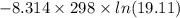
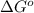 is equal to 7.309 kJ/mol.
is equal to 7.309 kJ/mol.



