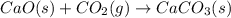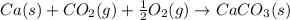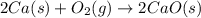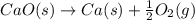Consider the equations below. (1) ca(s) + co2(g) + 1 2 o2(g) → caco3(s) (2) 2ca(s)+o2(g) → 2cao(s) how should you manipulate these equations so that they produce the equation below when added? check all that apply. cao(s) + co2(g) → caco3(s) reverse the direction of equation (2) multiply equation (1) by 3 multiply equation (2) by 1/2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
Do you know the correct answer?
Consider the equations below. (1) ca(s) + co2(g) + 1 2 o2(g) → caco3(s) (2) 2ca(s)+o2(g) → 2cao(s) h...
Questions in other subjects:




English, 01.07.2019 08:30





Social Studies, 01.07.2019 08:30