
Chemistry, 07.10.2019 22:00, alayciaruffin076
To use combustion analysis data to determine an empirical formula a molecular formula expresses the number of each kind of atom in a molecule. for example, the molecular formula for propene, c3h6, indicates three carbon atoms and six hydrogen atoms per molecule. this also means that one mole of propene contains three moles of carbon and six moles of hydrogen. an empirical formula expresses the mole ratio of the elements. the empirical formula for propene is ch2, indicating twice as much hydrogen as carbon. when analyzing unknown compounds in a lab, it is often possible to identify the mole ratios, and thus the empirical formula, but not the molecular formula. notice that the molecular mass of propene, 3(12)+6(1)=42amu, is a multiple of the empirical formula mass, 1(12)+2(1)=14amu . an unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 3.00 g of this compound produced 4.40 g of carbon dioxide and 1.80 g of water. how many moles of carbon, c, were in the original sample? how many moles of hydrogen, h, were in the original sample?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
Do you know the correct answer?
To use combustion analysis data to determine an empirical formula a molecular formula expresses the...
Questions in other subjects:





Mathematics, 03.04.2020 02:35



Mathematics, 03.04.2020 02:35

History, 03.04.2020 02:35


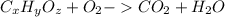
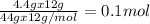 of Carbon
of Carbon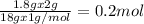 of Hydrogen
of Hydrogen




