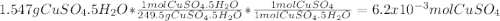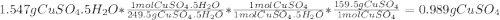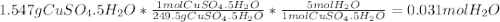Chemistry, 07.10.2019 19:10, jeffro198004
A1.547 g sample of blue copper(ii) sulfate pentahydrate, , is heated carefully to drive off the water. the white crystals of that are left behind have a mass of g. how many moles of were in the original sample? show that the relative molar amounts of and agree with the formula of the hydrate.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 08:00, kathrynpuppies201716
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1

Chemistry, 23.06.2019 08:10, andrewrangel63
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1
Do you know the correct answer?
A1.547 g sample of blue copper(ii) sulfate pentahydrate, , is heated carefully to drive off the wa...
Questions in other subjects:


Chemistry, 20.11.2020 18:40




History, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Arts, 20.11.2020 18:40


Biology, 20.11.2020 18:40


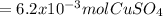
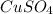 :
: