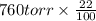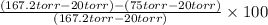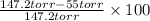Suppose that you are climbing a high mountain and the oxygen partial pressure in the air is reduced to 75 torr. estimate the percentage of the oxygen carrying capacity that will be utilized, assuming that the ph of both tissues and lungs is 7.4 and that the oxygen concentration in the tissues is 20 torr. show all work. i need to know how to work this problem out. the answer is 62.7%. show all work and i will award 5 stars.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1
Do you know the correct answer?
Suppose that you are climbing a high mountain and the oxygen partial pressure in the air is reduced...
Questions in other subjects:


Mathematics, 09.11.2019 02:31

Physics, 09.11.2019 02:31

Biology, 09.11.2019 02:31

Business, 09.11.2019 02:31

History, 09.11.2019 02:31





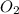 =
=