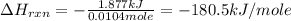Chemistry, 07.10.2019 16:20, chrismeldajbaptiste
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648 m hcl, also at 25.00°c. after stirring, the final temperature is 29.22°x. {assume that the total volume is the sum of the individual volumes and that the final solution has the same density (1.00 g/ml) and specific heat capacity (4.184 j/g°c) as water}. calculate the change in enthalpy, \deltaδh, of the reaction (in kj/mol) of water formed. enter the appropriate sign (+/ enter to 1 decimal place.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, netflixacc0107
Achemist requires 6.00 liters of 0.320 m h2so4 solution. how many grams of h2so4 should the chemist dissolve in water? 121 grams 159 grams 176 grams 188 grams
Answers: 2

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1
Do you know the correct answer?
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648...
Questions in other subjects:


Mathematics, 05.09.2020 03:01







Mathematics, 05.09.2020 03:01


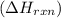 is, -180.5 kJ/mole
is, -180.5 kJ/mole
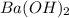
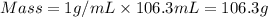
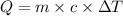
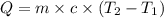
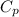 = specific heat capacity of water =
= specific heat capacity of water = 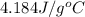
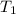 = initial temperature =
= initial temperature = 
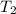 = final temperature =
= final temperature = 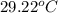
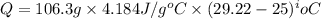
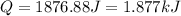 (1 kJ = 1000 J)
(1 kJ = 1000 J)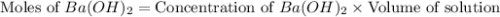
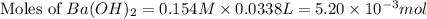
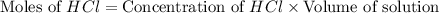
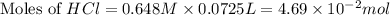
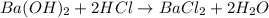
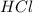
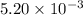 moles of
moles of 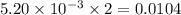 moles of
moles of 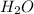
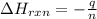
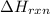 = enthalpy of reaction = ?
= enthalpy of reaction = ?