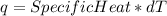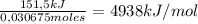Chemistry, 07.10.2019 16:10, dillondelellis2006
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose, c12h22o11, was burned in a bomb calorimeter with a heat capacity of 7.50 kj/oc (including its water). the temperature inside the calorimeter was found to increase by 20.2 oc. based on this information, what is the heat of this reaction per mole of sucrose?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
Do you know the correct answer?
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose...
Questions in other subjects:


SAT, 30.11.2021 04:10


Social Studies, 30.11.2021 04:10

History, 30.11.2021 04:10


Social Studies, 30.11.2021 04:10