
Chemistry, 07.10.2019 01:10, sandlobster6274
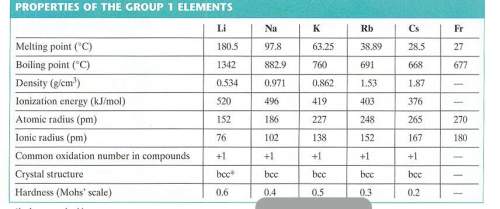
Use the radius of a rubidium atom from the table below to calculate the number of rubidium atoms in a row 6.00 cm long. assume that each rubidium atom touches the ones next to it.
atoms?

Answers: 1
Similar questions

Physics, 21.06.2019 16:10, lberman2005p77lfi
Answers: 1


Do you know the correct answer?
Use the radius of a rubidium atom from the table below to calculate the number of rubidium atoms in...
Questions in other subjects:





Mathematics, 25.06.2020 02:01












