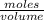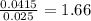Chemistry, 06.10.2019 10:02, Smartpotato9555
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1678 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrating it with the standard solution. the endpoint was reached after the addition of 19.88 ml of the standard solution. what is the concentration of the original sulfuric acid solution?

Answers: 1
Similar questions


Chemistry, 15.10.2019 18:00, kingalex7575
Answers: 2

Chemistry, 18.10.2019 01:30, vanessam16
Answers: 1

Chemistry, 30.10.2019 00:31, tyijiapostell
Answers: 2
Do you know the correct answer?
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standa...
Questions in other subjects:

History, 09.02.2021 15:10


Mathematics, 09.02.2021 15:10

Mathematics, 09.02.2021 15:10


Physics, 09.02.2021 15:10

Social Studies, 09.02.2021 15:10



Physics, 09.02.2021 15:10


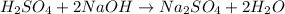
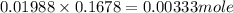
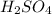 in 10 mL of diluted solution = 1/2 x moles of NaOH
in 10 mL of diluted solution = 1/2 x moles of NaOH
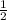 x 0.00333 = 0.00166 mol
x 0.00333 = 0.00166 mol
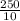 x 0.00166 = 0.0415 mol
x 0.00166 = 0.0415 mol