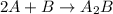Amultistep reaction can only occur as fast as its slowest step. therefore, it is the rate law of the slow step that determines the rate law for the overall reaction. consider the following multistep reaction: a + b → ab (slow) a + ab → a2b (fast)2a + b→ a2b (overall) based on this mechanism, determine the rate law for the overall reaction.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3


Chemistry, 22.06.2019 22:20, trockout4868
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
Do you know the correct answer?
Amultistep reaction can only occur as fast as its slowest step. therefore, it is the rate law of the...
Questions in other subjects:







Computers and Technology, 31.08.2020 02:01




![Rate=k[A][B]](/tpl/images/0288/1503/27e48.png)
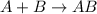 (slow)
(slow)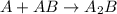 (fast)
(fast)