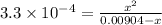The active ingredient in aspirin is acetylsalicylic acid (hc9h7o4), a monoprotic acid with a ka of 3.3×10−4 at 25 ∘c . you may want to reference (pages 680 - 690) section 16.6 while completing this problem. what is the ph of a solution obtained by dissolving two extra-strength aspirin tablets, containing 440 mg of acetylsalicylic acid each, in 270 ml of water? express your answer to two decimal places.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
Do you know the correct answer?
The active ingredient in aspirin is acetylsalicylic acid (hc9h7o4), a monoprotic acid with a ka of 3...
Questions in other subjects:

History, 02.02.2020 16:43



History, 02.02.2020 16:43

Mathematics, 02.02.2020 16:43






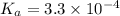
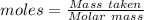
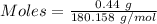
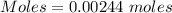
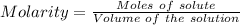
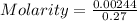
![K_{a}=\frac {\left [ H^{+} \right ]\left [ {C_9H_7O_4}^- \right ]}{[HC_9H_7O_4]}](/tpl/images/0288/1433/becfb.png)