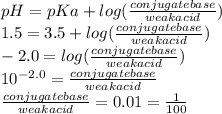Chemistry, 05.10.2019 04:10, meababy2009ow9ewa
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question. aspirin is a weak acid with a pka of 3.5 that is absorbed more effectively in the stomach than the small intestine. the ph of your stomach is around 1.5 and the ph of your small intestine is approximately 6.0. is aspirin absorbed more readily when it is protonated or deprotonated? what is the approximate ratio of conjugate base to acid when it is absorbed more readily?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
Do you know the correct answer?
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question...
Questions in other subjects:


Mathematics, 30.04.2021 20:20




Mathematics, 30.04.2021 20:20